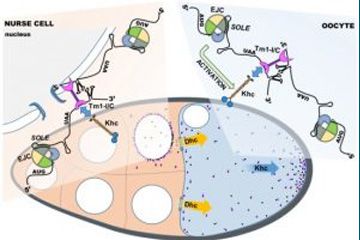
In this study, the Ephrussi lab shows that an atypical tropomyosin isoform is a direct (m)RNA binding protein that binds preferentially to the dimerizing oskar 3’ UTR and is a component of the transported oskar mRNPs within the female germ-line. In the absence of this tropomyosin isoform, Khc fails to get loaded onto oskar mRNA, which explains the reduced motility and ultimately the failure in oskar localization. This Tm1-I/C dependent recruitment is rather inefficient - only a small fraction of oskar mRNPs acquire Khc – but dynamic, enabling the posterior-ward transport of virtually all oskar mRNPs. Most importantly, however, the Tm1-I/C recruited Khc is inactive. Activation of the motor only commences in the oocyte during mid-oogenesis – possibly to prevent interference with the other transporter of oskar, cytoplasmic dynein - and requires the previously identified exon junction complex (EJC) and associated spliced oskar localization element (SOLE).
Gaspar, I., Sysoev, V., Komissarov, A. and Ephrussi, A. (2016)
An RNA-binding atypical tropomyosin recruits kinesin-1 dynamically to oskar mRNPs.
EMBO J. DOI 10.15252/embj.201696038
Forschergruppe 2333
Prof. Dr. Dierk Niessing (Sprecher)
Ulm University - Institute of Pharmaceutical Biotechnology
James Franck Ring N27
89081 Ulm
Phone: +49 89 3187 2176
EMail: info@for2333.de